How Do You Know if Its a Cation or Anion
Chemical Reactivity |
|---|
Chemical Reactivity
Organic chemistry encompasses a very large number of compounds ( many millions ), and our previous give-and-take and illustrations take focused on their structural characteristics. Now that nosotros can recognize these actors ( compounds ), nosotros turn to the roles they are inclined to play in the scientific drama staged by the multitude of chemic reactions that define organic chemistry.
We begin by defining some basic terms that volition be used oft as this field of study is elaborated.
Chemical Reaction: A transformation resulting in a modify of composition, constitution and/or configuration of a compound ( referred to as the reactant or substrate ).
Reactant or Substrate: The organic compound undergoing change in a chemical reaction. Other compounds may besides exist involved, and mutual reactive partners ( reagents ) may be identified. The reactant is oftentimes ( but non always ) the larger and more complex molecule in the reacting system. About ( or all ) of the reactant molecule is ordinarily incorporated every bit function of the product molecule.
Reagent: A common partner of the reactant in many chemical reactions. It may be organic or inorganic; small or large; gas, liquid or solid. The portion of a reagent that ends up being incorporated in the product may range from all to very little or none.
Production(southward) The final form taken by the major reactant(south) of a reaction.
Reaction Weather The environmental conditions, such as temperature, force per unit area, catalysts & solvent, under which a reaction progresses optimally. Catalysts are substances that accelerate the rate ( velocity ) of a chemic reaction without themselves being consumed or appearing every bit office of the reaction production. Catalysts do not modify equilibria positions.
Chemical reactions are normally written as equations: | 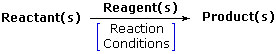 |
|---|
Reaction Nomenclature |
|---|
Classifying Organic Chemical Reactions
If you lot browse whatsoever organic textbook you volition encounter what appears to be a very big, often intimidating, number of reactions. These are the "tools" of a chemist, and to use these tools finer, nosotros must organize them in a sensible manner and look for patterns of reactivity that permit us make plausible predictions. Most of these reactions occur at special sites of reactivity known equally functional groups, and these constitute one organizational scheme that helps the states catalog and remember reactions.
Ultimately, the all-time way to achieve proficiency in organic chemistry is to sympathize how reactions take place, and to recognize the various factors that influence their course.
This is all-time achieved past perceiving the reaction pathway or mechanism of a reaction.
i. Classification by Structural Change
Get-go, we identify 4 broad classes of reactions based solely on the structural change occurring in the reactant molecules. This nomenclature does non require knowledge or speculation concerning reaction paths or mechanisms.
The letter R in the following illustrations is widely used as a symbol for a generic group. It may represent simple substituents such as H– or CH3–, or for complex groups composed of many atoms of carbon and other elements.
Four Reaction Classes | ||
|---|---|---|
Addition | Elimination | |
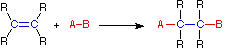 | 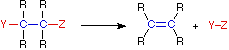 | |
Substitution | Rearrangement | |
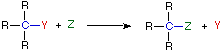 | 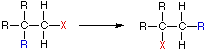 | |
In an addition reaction the number of σ-bonds in the substrate molecule increases, commonly at the expense of one or more π-bonds. The reverse is truthful of emptying reactions, i.e.the number of σ-bonds in the substrate decreases, and new π-bonds are oftentimes formed. Substitution reactions, every bit the name implies, are characterized by replacement of an atom or group (Y) by another atom or group (Z). Bated from these groups, the number of bonds does not change. A rearrangement reaction generates an isomer, and again the number of bonds normally does not modify.
The examples illustrated to a higher place involve simple alkyl and alkene systems, just these reaction types are full general for most functional groups, including those incorporating carbon-oxygen double bonds and carbon-nitrogen double and triple bonds. Some common reactions may actually be a combination of reaction types. The reaction of an ester with ammonia to give an amide, as shown below, appears to be a substitution reaction ( Y = CH3O & Z = NHii ); however, information technology is actually 2 reactions, an improver followed past an elimination.
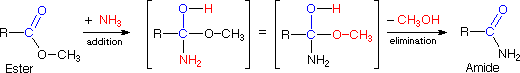
The add-on of water to a nitrile does not seem to fit any of the in a higher place reaction types, but information technology is merely a wearisome add-on reaction followed by a rapid rearrangement, as shown in the following equation. Rapid rearrangements of this kind are chosen tautomerizations.
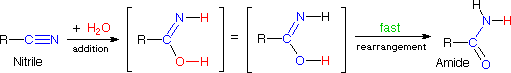
Additional examples illustrating these classes of reaction may be examined past Clicking Here
2. Classification by Reaction Type
At the offset, it is helpful to identify some common reaction types that will surface repeatedly as the chemical behavior of different compounds is examined. This is not intended to be a complete and comprehensive list, just should gear up the phase for future elaborations.
Acidity and Basicity
It is useful to begin a discussion of organic chemical reactions with a review of acid-base chemistry and terminology for several reasons. First, acrid-base reactions are amidst the simplest to recognize and understand. Second, some classes of organic compounds take distinctly acidic properties, and some other classes behave every bit bases, so we need to place these aspects of their chemistry. Finally, many organic reactions are catalyzed past acids and/or bases, and although such transformations may seem complex, our understanding of how they occur often begins with the performance of the goad.
Organic chemists employ two acid-base theories for interpreting and planning their work: the Brønsted theory and the Lewis theory .
Brønsted Theory
According to the Brønsted theory, an acid is a proton donor, and a base is a proton acceptor. In an acid-base of operations reaction, each side of the equilibrium has an acid and a base of operations reactant or product, and these may be neutral species or ions.
| H-A + B:(–) |  | A:(–) + B-H |
| (acid1) (base1) | (base2) (acidtwo) |
Structurally related acrid-base pairs, such equally {H-A and A:(–)} or {B:(–) and B-H} are called conjugate pairs. Substances that tin can serve every bit both acids and bases, such as water, are termed amphoteric.
| H-Cl + H2O |  | Cl:(–) + H3O(+) |
| (acrid) (base) | (base) (acid) |
| H3N: + HtwoO |  | NHfour (+) + HO(–) |
| (base) (acid) | (acid) (base) |
The relative strength of a group of acids (or bases) may be evaluated past measuring the extent of reaction that each group member undergoes with a common base (or acid). H2o serves nicely every bit the common base or acid for such determinations. Thus, for an acid H-A, its strength is proportional to the extent of its reaction with the base h2o, which is given by the equilibrium constant Keq.
| H-A + H2O |  | HiiiO(+) + A: (–) | 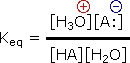 |
Since these studies are generally extrapolated to high dilution, the molar concentration of water (55.five) is constant and may be eliminated from the denominator. The resulting 1000 value is called the acidity constant, Ka. Clearly, stiff acids accept larger Ka's than do weaker acids. Considering of the very large range of acid strengths (greater than 1040), a logarithmic scale of acidity (pKa) is usually employed. Stronger acids have smaller or more negative pKa values than exercise weaker acids.
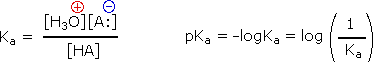
| Some useful principles of acid-base reactions are: |
|---|
Examples of Brønsted Acid-Base Equilibria
| Acid-Base of operations Reaction | Conjugate Acids | Cohabit Bases | Thoua | pKa | |||
|---|---|---|---|---|---|---|---|
| HBr + H2O |  | H3O(+) + Br(–) | HBr H3O(+) | Br(–) H2O | 10v | -5 | |
| CH3CO2H + H2O |  | HthreeO(+) + CHiiiCO2 (–) | CH3CO2H HthreeO(+) | CHiiiCOii (–) H2O | 1.77*ten-5 | 4.75 | |
| CtwoHvOH + H2O |  | H3O(+) + C2HfiveO(–) | C2HvOH H3O(+) | C2H5O(–) HtwoO | 10-16 | 16 | |
| NH3 + H2O |  | H3O(+) + NHtwo (–) | NH3 HiiiO(+) | NH2 (–) HtwoO | 10-34 | 34 | |
In all the to a higher place examples water acts as a common base. The last example ( NH3 ) cannot be measured directly in water, since the strongest base that can exist in this solvent is hydroxide ion. Consequently, the value reported hither is extrapolated from measurements in much less acidic solvents, such as acetonitrile.
Since many organic reactions either take place in aqueous environments ( living cells ), or are quenched or worked-upwards in water, it is important to consider how a conjugate acrid-base of operations equilibrium mixture changes with pH. A simple relationship known as the Henderson-Hasselbalch equation provides this information.
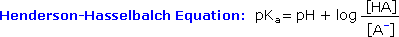
When the pH of an aqueous solution or mixture is equal to the pKa of an acidic component, the concentrations of the acrid and base of operations conjugate forms must be equal ( the log of ane is 0 ). If the pH is lowered by ii or more units relative to the pKa, the acid concentration will be greater than 99%. On the other manus, if the pH ( relative to pKa ) is raised by two or more units the cohabit base concentration will be over 99%. Consequently, mixtures of acidic and non-acidic compounds are easily separated past adjusting the pH of the water component in a two phase solvent extraction.
For case, if a solution of benzoic acid ( pKa = four.ii ) in benzyl alcohol ( pKa = 15 ) is dissolved in ether and shaken with an excess of 0.1 Due north sodium hydroxide ( pH = 13 ), the acid is completely converted to its water soluble ( ether insoluble ) sodium common salt, while the alcohol is unaffected. The ether solution of the booze may then be separated from the water layer, and pure alcohol recovered past distillation of the volatile ether solvent. The pH of the water solution of sodium benzoate may then be lowered to 1.0 past addition of hydrochloric acid, at which bespeak pure benzoic acid crystallizes, and may be isolated by filtration.
| For a discussion of how acerbity is influenced by molecular construction Click Hither. |
|---|
Basicity
The basicity of oxygen, nitrogen, sulfur and phosphorus compounds or ions may be treated in an analogous fashion. Thus, we may write base-acrid equilibria, which define a Kb and a corresponding pKb. However, a more common procedure is to written report the acidities of the conjugate acids of the bases ( these cohabit acids are often "onium" cations ). The pKa's reported for bases in this system are proportional to the base strength of the base. A useful rule here is: pKa + pKb = fourteen.
We run across this relationship in the following two equilibria:
| Acid-Base Reaction | Conjugate Acids | Conjugate Bases | K | pK | |||
|---|---|---|---|---|---|---|---|
| NH3 + H2O |  | NH4 (+) + OH(–) | NHiv (+) H2O | NH3 OH(–) | Mb = 1.8*x-v | pKb = 4.74 | |
| NHfour (+) + HiiO |  | H3O(+) + NH3 | NHiv (+) H3O(+) | NH3 H2O | Granda = v.5*10-10 | pKa = ix.25 | |
Tables of pKa values for inorganic and organic acids ( and bases) are available in many reference books, and may exist examined here past clicking on the advisable link:
Although information technology is convenient and informative to limited pKa values for a mutual solvent system (ordinarily h2o), there are serious limitations for very strong and very weak acids. Thus acids that are stronger than the hydronium cation, HiiiO(+), and weak acids having conjugate bases stronger than hydroxide anion, OH(–), cannot be measured straight in h2o solution. Solvents such as acerb acid, acetonitrile and nitromethane are often used for studying very strong acids. Relative acidity measurements in these solvents may exist extrapolated to water. Likewise, very weakly acidic solvents such every bit DMSO, acetonitrile, toluene, amines and ammonia may exist used to study the acidities of very weak acids. For both these groups, the reported pKa values extrapolated to water are estimate, and many take large uncertainties. A useful table of pKa values in DMSO solution has been compiled from the piece of work of F.G. Bordwell, and may be reached past Clicking Here.
Lewis Theory
According to the Lewis theory, an acrid is an electron pair acceptor, and a base is an electron pair donor. Lewis bases are likewise Brønsted bases; even so, many Lewis acids, such as BF3, AlCl3 and Mg2+, are non Brønsted acids. The product of a Lewis acid-base reaction, is a neutral, dipolar or charged complex, which may be a stable covalent molecule. As shown at the top of the following drawing, coordinate covalent bonding of a phosphorous Lewis base of operations to a boron Lewis acrid creates a complex in which the formal charge of boron is negative and that of phosphorous is positive. In this complex, boron acquires a neon valence crush configuration and phosphorous an argon configuration. If the substituents (R) on these atoms are not big, the complex will be favored at equilibrium. Yet, steric hindrance of bulky substituents may prohibit complex formation. The resulting mixture of non-bonded Lewis acid/base pairs has been termed "frustrated", and exhibits unusual chemical behavior.
Two examples of Lewis acid-base equilibria that play a part in chemical reactions are shown in equations 1 & 2 beneath.
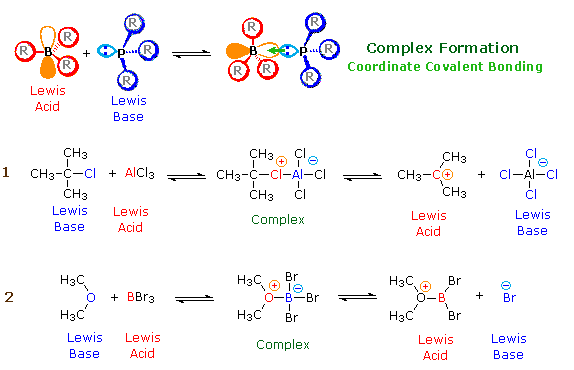
In the first case, an electron scarce aluminum cantlet bonds to a covalent chlorine cantlet by sharing one of its non-bonding valence electron pairs, and thus achieves an argon-like valence shell octet. Because this sharing is unilateral (chlorine contributes both electrons), both the aluminum and the chlorine have formal charges, every bit shown. If the carbon chlorine bail in this circuitous breaks with both the bonding electrons remaining with the more electronegative atom (chlorine), the carbon assumes a positive charge. We refer to such carbon species as carbocations. Carbocations are too Lewis acids, every bit the contrary reaction demonstrates.
Many carbocations (only not all) may also function as Brønsted acids. Equation 3 illustrates this dual behavior; the Lewis acidic site is colored red and iii of the nine acidic hydrogen atoms are colored orange. In its Brønsted acid function the carbocation donates a proton to the base (hydroxide anion), and is converted to a stable neutral molecule having a carbon-carbon double bond.
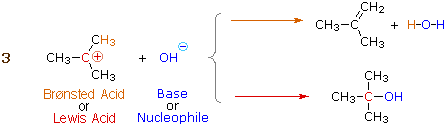
A terminology related to the Lewis acid-base classification is often used by organic chemists. Here the term electrophile corresponds to a Lewis acid, and nucleophile corresponds to a Lewis base.
Electrophile : An electron scarce atom, ion or molecule that has an analogousness for an electron pair, and volition bond to a base or nucleophile.
Nucleophile : An cantlet, ion or molecule that has an electron pair that may be donated in bonding to an electrophile (or Lewis acrid).
| To learn more about the human relationship of basicity and nucleophilicity, |
|---|
Oxidation and Reduction Reactions
A parallel and independent method of characterizing organic reactions is by oxidation-reduction terminology. Carbon atoms may have any oxidation state from –iv (east.g. CHiv ) to +4 (eastward.m. CO2 ), depending upon their substituents. Fortunately, we demand not determine the absolute oxidation country of each carbon atom in a molecule, but simply the change in oxidation country of those carbons involved in a chemical transformation. To determine whether a carbon cantlet has undergone a redox change during a reaction we simply note any changes in the number of bonds to hydrogen and the number of bonds to more than electronegative atoms such as O, N, F, Cl, Br, I, & Southward that has occurred. Bonds to other carbon atoms are ignored. This count should be conducted for each carbon atom undergoing any change during a reaction.
-
If the number of hydrogen atoms bonded to a carbon increases, and/or if the number of bonds to more electronegative atoms decreases, the carbon in question has been reduced (i.e. it is in a lower oxidation country).
-
If the number of hydrogen atoms bonded to a carbon decreases, and/or if the number of bonds to more electronegative atoms increases, the carbon in question has been oxidized (i.e. information technology is in a higher oxidation state).
-
If there has been no change in the number of such bonds, and then the carbon in question has not changed its oxidation state. In the hydrolysis reaction of a nitrile shown higher up, the blue colored carbon has not inverse its oxidation state.
These rules are illustrated by the post-obit four addition reactions involving the same starting material, cyclohexene. Carbon atoms colored blue are reduced, and those colored crimson are oxidized. In the improver of hydrogen both carbon atoms are reduced, and the overall reaction is termed a reduction. Peracid epoxidation and addition of bromine oxidize both carbon atoms, so these are termed oxidation reactions. Addition of HBr reduces one of the double bond carbon atoms and oxidizes the other; consequently, there is no overall redox change in the substrate molecule.
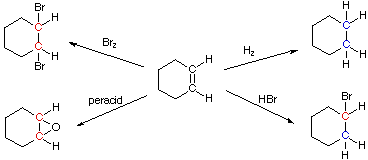
| For a discussion of how oxidation state numbers may be assigned to carbon atoms Click Here. |
|---|
Since metals such every bit lithium and magnesium are less electronegative than hydrogen, their covalent bonds to carbon are polarized then that the carbon is negative (reduced) and the metallic is positive (oxidized). Thus, Grignard reagent formation from an alkyl halide reduces the substituted carbon atom. In the post-obit equation and half-reactions the carbon atom (blue) is reduced and the magnesium (magenta) is oxidized.
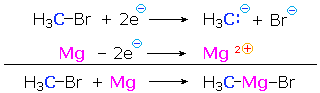
iii. Classification by Functional Group
Functional groups are atoms or small groups of atoms (unremarkably two to four) that exhibit a characteristic reactivity when treated with certain reagents. To view a table of the common functional groups and their grade names Click Here. A particular functional group will almost always display its feature chemical behavior when it is present in a compound. Because of this, the discussion of organic reactions is oft organized according to functional groups. The post-obit table summarizes the general chemical behavior of the common functional groups. For reference, the alkanes provide a background of beliefs in the absence of more than localized functional groups.
| Functional Class | Formula | Characteristic Reactions |
|---|---|---|
| Alkanes | C–C, C–H | Substitution (of H, commonly by Cl or Br) Combustion (conversion to CO2 & H2O) |
| Alkenes | C=C–C–H | Addition Commutation (of H) |
| Alkynes | C≡C–H | Add-on Commutation (of H) |
| Alkyl Halides | H–C–C–X | Substitution (of X) Emptying (of HX) |
| Alcohols | H–C–C–O–H | Commutation (of H); Substitution (of OH) Elimination (of HOH); Oxidation (elimination of 2H) |
| Ethers | (α)C–O–R | Substitution (of OR); Substitution (of α–H) |
| Amines | C–NRH | Substitution (of H); Add-on (to North); Oxidation (of N) |
| Benzene Ring | C6H6 | Exchange (of H) |
| Aldehydes | (α)C–CH=O | Add-on Substitution (of H or α–H) |
| Ketones | (α)C–CR=O | Addition Substitution (of α–H) |
| Carboxylic Acids | (α)C–COtwoH | Exchange (of H); Substitution (of OH) Substitution (of α–H); Improver (to C=O) |
| Carboxylic Derivatives | (α)C–CZ=O (Z = OR, Cl, NHR, etc.) | Substitution (of Z); Substitution (of α–H) Addition (to C=O) |
This table does not include any reference to rearrangement, due to the fact that such reactions are found in all functional classes, and are highly dependent on the structure of the reactant. Furthermore, a review of the overall reaction patterns presented in this table discloses only a broad and rather non-specific gear up of reactivity trends. This is non surprising, since the three remaining categories provide only a coarse discrimination (comparable to identifying an object as animal, vegetable or mineral). Consequently, credible similarities may fail to reflect important differences. For example, add-on reactions to C=C are significantly different from additions to C=O, and substitution reactions of C-X proceed in very different ways, depending on the hybridization state of carbon.
Reaction Variables |
|---|
The Variables of Organic Reactions
In an effort to understand how and why reactions of functional groups take identify in the way they do, chemists try to discover just how different molecules and ions interact with each other as they come together. To this terminate, it is important to consider the diverse properties and characteristics of a reaction that may be observed and/or measured as the reaction gain . The nigh common and useful of these are listed beneath:
1. Reactants and Reagents
| A. Reactant Structure: Variations in the structure of the reactant may have a marked influence on the course of a reaction, even though the functional group is unchanged. Thus, reaction of one-bromopropane with sodium cyanide proceeds smoothly to yield butanenitrile, whereas 1-bromo-ii,2-dimethylpropane fails to give whatever product and is recovered unchanged. In contrast, both alkyl bromides form Grignard reagents (RMgBr) on reaction with magnesium. | |
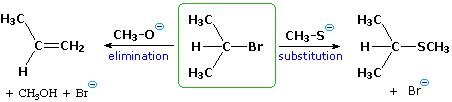
| B. Reagent Characteristics: Plain small-scale changes in a reagent may atomic number 82 to a significant change in the course of a reaction. For example, 2-bromopropane gives a commutation reaction with sodium methylthiolate but undergoes predominant elimination on handling with sodium methoxide. |
2. Product Selectivity
| A. Regioselectivity: It is often the case that addition and elimination reactions may, in principle, continue to more than than one product. Thus 1-butene might add HBr to give either one-bromobutane or 2-bromobutane, depending on which carbon of the double bond receives the hydrogen and which the bromine. If one possible product out of ii or more is formed preferentially, the reaction is said to be regioselective. Simple commutation reactions are non ordinarily considered regioselective, since past definition but one constitutional product is possible. However, rearrangements are known to occur during some reactions. | |
| B. Stereoselectivity: If the reaction products are such that stereoisomers may be formed, a reaction that yields one stereoisomer preferentially is said to be stereoselective. In the improver of bromine to cyclohexene, for case, cis and trans-1,ii-dibromocyclohexane are both possible products of the addition. Since the trans-isomer is the only isolated product, this reaction is stereoselective. | |
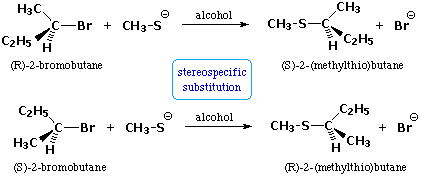
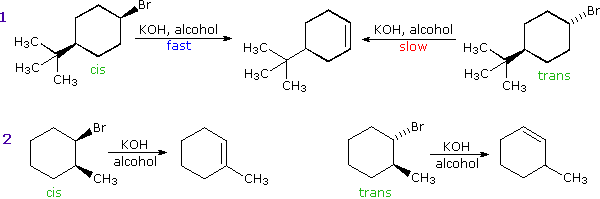
| C. Stereospecificity: This term is applied to cases in which stereoisomeric reactants behave differently in a given reaction. Examples include: Here, the (R)-reactant gives the configurationally inverted (S)-product, and (S)-reactant produces (R)-product. The (R) and (S) notations for configuration are described in a afterwards section of this text. (2) Unlike rates of reaction, equally in the base of operations-induced elimination of cis & trans-iv-tert-butylcyclohexyl bromide (equation 1 beneath). (iii) Different reaction paths leading to different products, as in the base-induced emptying of cis & trans-two-methylcyclohexyl bromide (equation ii below). The mechanisms of these substitution and elimination reactions are discussed in the alkyl halide department of this text. |
3. Reaction Characteristics
| A. Reaction Rates: Some reactions proceed very rapidly, and some so slowly that they are non normally observed. Among the variables that influence reaction rates are temperature (reactions are usually faster at a higher temperature), solvent, and reactant / reagent concentrations. Useful information about reaction mechanisms may be obtained by studying the manner in which the rate of a reaction changes as the concentrations of the reactant and reagents are varied. This subject field is called kinetics. | |
| B. Intermediates: Many reactions keep in a stepwise way. This tin be assuredly demonstrated if an intermediate species tin can be isolated and shown to go on to the same products under the reaction conditions. Some intermediates are stable compounds in their own right; however, some are so reactive that isolation is not possible. However, evidence for their beingness may exist obtained past other means, including spectroscopic ascertainment or inference from kinetic results. |
four. Factors that Influence Reactions
It is helpful to identify some general features of a reaction that accept a pregnant influence on its facility. Some of the most important of these are:
| A. Energetics: The potential energy of a reacting organization changes as the reaction progresses. The overall change may be exothermic ( energy is released ) or endothermic ( energy must be added ), and in that location is usually an activation energy requirement as well. Tables of Standard Bond Energies are widely used by chemists for estimating the energy change in a proposed reaction. Equally a rule, compounds constructed of stiff covalent bonds are more stable than compounds incorporating i or more relatively weak bonds. | |
| B. Electronic Effects: The distribution of electrons at sites of reaction (functional groups) is a specially important factor. Electron deficient species or groups, which may or may not exist positively charged, are attracted to electron rich species or groups, which may or may not be negatively charged. Nosotros refer to these species equally electrophiles & nucleophiles respectively. In general, opposites concenter and like repel . | |
| C. Steric Effects: Atoms occupy space. When they are crowded together, van der Waals repulsions produce an unfavorable steric hindrance. Steric hindrance may influence conformational equilibria, as well as destabilizing transition states of reactions. | |
| D. Stereoelectronic Effects: In many reactions atomic or molecular orbitals collaborate in a fashion that has an optimal configurational or geometrical alignment. Deviation from this alignment inhibits the reaction. | |
| E. Solvent Effects: Most reactions are conducted in solution, not in a gaseous state. The solvent selected for a given reaction may exert a strong influence on its form. Recall, solvents are chemicals, and most undergo chemic reaction under the right conditions. |
Reaction Mechanisms |
|---|
Mechanisms of Organic Reactions
A detailed description of the changes in structure and bonding that take place in the course of a reaction, and the sequence of such events is called the reaction mechanism. A reaction mechanism should include a representation of plausible electron reorganization, too as the identification of any intermediate species that may be formed as the reaction progresses. These features are elaborated in the following sections.
1. The Arrow Note in Mechanisms
Since chemical reactions involve the breaking and making of bonds, a consideration of the movement of bonding ( and non-bonding ) valence shell electrons is essential to this understanding. Information technology is now common exercise to show the motion of electrons with curved arrows, and a sequence of equations depicting the consequences of such electron shifts is termed a mechanism. In general, ii kinds of curved arrows are used in cartoon mechanisms:
The use of these symbols in bond-breaking and bond-making reactions is illustrated below. If a covalent single bond is broken and so that 1 electron of the shared pair remains with each fragment, as in the kickoff example, this bond-breaking is called homolysis. If the bond breaks with both electrons of the shared pair remaining with i fragment, as in the second and tertiary examples, this is called heterolysis.
Other Arrow Symbols
Chemists also use arrow symbols for other purposes, and it is essential to use them correctly.
| The Reaction Arrow | The Equilibrium Arrow | The Resonance Arrow |
|---|---|---|
 |  |  |
The following equations illustrate the proper use of these symbols:
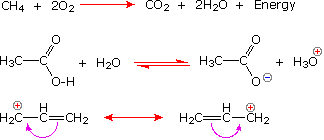
| For further data about the use of curved arrows in reaction mechanisms Click Here. |
|---|
two. Reactive Intermediates
The products of bond breaking, shown above, are non stable in the usual sense, and cannot be isolated for prolonged written report. Such species are referred to equally reactive intermediates, and are believed to be transient intermediates in many reactions. The general structures and names of four such intermediates are given below.
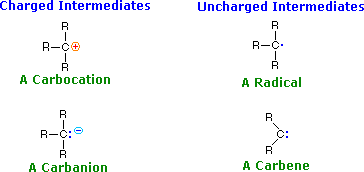
A pair of widely used terms, related to the Lewis acid-base note, should also exist introduced here.
| Electrophile: An electron scarce cantlet, ion or molecule that has an affinity for an electron pair, and will bond to a base or nucleophile. |
Using these definitions, information technology is clear that carbocations ( called carbonium ions in the older literature ) are electrophiles and carbanions are nucleophiles. Carbenes have simply a valence shell sextet of electrons and are therefore electron scarce. In this sense they are electrophiles, merely the non-bonding electron pair also gives carbenes nucleophilic grapheme. As a rule, the electrophilic graphic symbol dominates carbene reactivity. Carbon radicals have only seven valence electrons, and may be considered electron deficient; yet, they practise non in full general bond to nucleophilic electron pairs, so their chemistry exhibits unique differences from that of conventional electrophiles. Radical intermediates are often chosen free radicals.
The importance of electrophile / nucleophile terminology comes from the fact that many organic reactions involve at some stage the bonding of a nucleophile to an electrophile, a procedure that mostly leads to a stable intermediate or production. Reactions of this kind are sometimes chosen ionic reactions, since ionic reactants or products are often involved. Some common examples of ionic reactions and their mechanisms may exist examined past Clicking Here
The shapes ideally assumed by these intermediates becomes important when considering the stereochemistry of reactions in which they play a function. A uncomplicated tetravalent compound like methane, CHfour, has a tetrahedral configuration. Carbocations have only three bonds to the accuse begetting carbon, so it adopts a planar trigonal configuration. Carbanions are pyramidal in shape ( tetrahedral if the electron pair is viewed as a substituent ), but these species invert rapidly at room temperature, passing through a higher energy planar form in which the electron pair occupies a p-orbital. Radicals are intermediate in configuration, the free energy divergence betwixt pyramidal and planar forms beingness very small. Since three points decide a plane, the shape of carbenes must exist planar; however, the valence electron distribution varies.
Practise Problems |
|---|
| The following problems include acid base relationships, recognition of unlike functional groups, recognition of nucleophiles and electrophiles, nomenclature of reactions by structural modify and oxidation/reduction alter, and the utilize of curved arrow notation. |
| Return to Table of Contents |
|---|
This page is the belongings of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to help in capacity building in chemical education. 05/05/2013
![]()
Source: https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/react1.htm
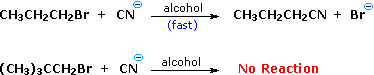

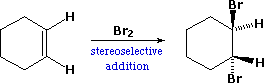
0 Response to "How Do You Know if Its a Cation or Anion"
Postar um comentário